Prof Salim Abdool Karim Weekly COVID-19 UPDATES
So, let’s start with the global situation (Figure 1). Yesterday, there were about 200,310 reported cases and 2,127 deaths across the world. There are still over 200,000 reported cases per day (the actual cases is much higher) and deaths have risen slightly about 10% in the last week. The map on the right side of Figure 1 shows that Japan has the largest increase in reported cases in the last month.

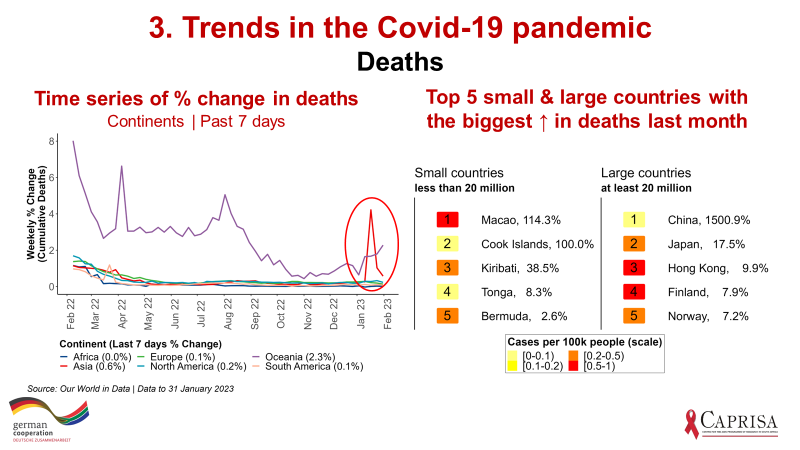
In Figure 2 below, I have provided the 5 small countries (population < 20 million) and the 5 large countries (population > 20 million) that are the biggest contributors to new cases over the last 7 days. Note that the colour code on the number indicates the incidence rate ie. those that are yellow are large increases off a low base while red indicates both large numbers of confirmed cases and high incidence rates. Similar to what we saw last week, Japan, Taiwan, Hong Kong, South Korea and New Zealand have both rising numbers of cases and high incidence rates. Figure 3 provides similar data for deaths. As I pointed out last week, the lack of data from China is a concern, the spike in deaths in Figure 3 is a result of China providing some new interim data on deaths.


That was a quick snapshot of the global Covid-19 situation. Now to South Africa. The country remains in low transmission, though small outbreaks are continuing to occur (Figure 5). These localised transmissions are resulting in small increases in cases. Deaths have also increased slightly, though off a low base.
Given the low levels of testing in South Africa at present, it is more useful to look at the wastewater surveillance. When I get the wastewater data, I will include them in my email. In settings like South Africa where testing is low, there is good case to be made for sentinel surveillance for SARS-CoV-2 infection. A small number of sentinel sites could provide useful trend data on new infections and importantly provide positive swabs to enhance genomic surveillance for new variants.

After a concerningly long delay, the South African medicines regulator (SAHPRA) approved Nirmatrelvir/Ritonavir (Paxlovid) for the treatment of Covid-19 this week. FDA Emergency Use Authorization (EUA) was obtained in December 2021, ie. more than a year ago. The results of the original clinical trial were published in the NEJM in April 2022 (Figure 6). A 5-day course of Paxlovid started within 3 days of initial diagnosis reduces severe Covid-19 by 89%. Paxlovid creates in essence a Test & Treat strategy to reduce Covid-19 hospitalisations and deaths. On 6 July 2022, the FDA authorized pharmacists to prescribe Paxlovid to eligible patients, with some restrictions, to facilitate wider implementation of the Test & Treat approach.

One of the limitations of the original trial is that only unvaccinated patients were included. A real-world implementation study in a largely vaccinated population was conducted in Israel (Figure 7). The study found high efficacy of Paxlovid – preventing about 3 out of 4 hospitalisations and deaths, but only in elderly people above 64 years old (Figure 7). There was no discernible benefit in those 40-64 years. Hence, Paxlovid is mainly prescribed in elderly people who has have tested positive for Covid-19.
It is the standard of care in the USA, UK, Europe and most developed countries. It is not available in most middle-and-lower-income countries, reflecting global treatment inequity. It mirrors the inequity we witnessed pre-2000 when life-saving treatments for AIDS were only available in the developed world. But, unlike AIDS, there has been no outcry over Covid-19 treatment inequity, possible because the treatment’s benefits are not well known.

While Paxlovid has been shown to be highly efficacious in clinical trials and in real world implementation studies, there are significant limitations of this treatment. The first is that need to test to diagnose their SARS-CoV-2 infection to qualify for Paxlovid. And after testing, they need to start treatment within 3 days. As the benefits of Paxlovid gain traction, Covid-19 testing rates may improve because of the availability of treatment. We certainly saw this with HIV – when treatment became available, willingness to test and testing rates rose.
Second, Paxlovid gained media attention when President Biden took it when he had Covid-19. Dr Tony Fauci also took it when he had Covid-19. Both of them experienced rebound, ie. they tested Covid+ shortly after treatment completion ie. the antiviral effects of the drug wear off. The FDA has asked Pfizer to investigate whether a longer course can avert the problem of rebound.
Third, there are many other drugs that are metabolised by the enzyme pathway that is inhibited by Paxlovid – this drug-drug interaction requires checking for concomitant medication and adjustment of dosages or even temporary stoppages of other medications (Figure 8). This problem is, however, not as big as it may first appear. According to the Infectious Diseases Society of the USA, of the 100 or so drugs where drug-drug interactions are possible, only 2 - rivaroxaban and salmeterol - produce severe interactions. Patients taking either of these 2 drugs should avoid Paxlovid.
A fourth concern is around drug resistance, but is a challenge for every new antibiotic or anti-viral and is not grounds for not using an effective treatment.
The fifth concern about Paxlovid is cost – the price in the USA for $53 per dose ie. $530 for a course of treatment. However, in most developed countries, including the USA and Canada, the cost is fully covered by the government and Paxlovid is provided free for all patients. Generic versions of Paxlovid have been manufactured by pharmaceutical companies in India and they sell Paxlovid for about $2 per pill, ie. $20 for a course of treatment currently. These costs are expected to drop when widespread use leads to large volume production.

At $20 per course of treatment, Paxlovid is readily affordable in South Africa. A course of Paxlovid would be cheaper than the cost of the PCR test! In the elderly, where risk of hospitalisation and death is higher, it would be important to empirically assess the number of patients that need to be treated to prevent one hospitalisation or one death. If anyone has seen these estimates in any setting, please share them with me.
At US prices, the US Institute for Clinical and Economic Review calculated Paxlovid treatment to be cost-effective in the US.
But we should expect some resistance to the use of Paxlovid, especially from a handful of doctors. This is not unusual. It has been well documented in the USA and elsewhere. The New York Times published an article this week outlining the challenges in rolling out free Paxlovid in the US (Figure 9). A summary of the article is in Figure 9. For those interested in reading The New York Times article, An Underused Covid Treatment

I, for one, do not accept that poor countries are being deprived of a life-saving treatment that is strongly recommended by WHO and is the standard of care in wealthy countries. The WHO guidelines include a strong recommendation for Paxlovid for mild and moderate COVID-19 patients at highest risk of hospital admission (the elderly and those with co-morbidities). In my view, it is the best therapeutic choice for high-risk patients to date and if I ever get Covid-19, I will be taking Paxlovid!
Have a great week.


